In vitro imaging: Microscopy, Histology and Cytometry Services
Imaging techniques make it possible to observe organisms' functions at a molecular, cellular, or tissue level by more or less invasive means. From the observation of living cells using fluorescent molecular markers to the labeling of tissues with specific antibodies, imaging techniques allow the biology of an organism to be visualized precisely.
The observation of the biology of organisms is done at several levels:
- At the molecular level, observation is typically done by immunological labeling of molecules of interest, followed by observation by flow cytometry or microscopy.
- At the cellular level, by labeling different organelles or by observation of the state of the cell, for example by flow cytometry.
- At the tissue level, typically by histology, following different markings of cellular and molecular structures.
- At the level of the whole organism, using electron microscopy techniques after treatment of the organism, or imaging techniques on living organisms.
Microscopy has evolved on two levels: on the level of magnification, optical technologies can go to x 1,000 and transmission electron microscopes up to x 1,200,000; on the level of the markings of interest, by the use of fluorescence, specific markers, and immunological detection.
Histology has evolved notably through the acquisition of knowledge by anatomopathologists on the analysis of human and other tissue sections.
Microfluidics is used for flow cytometry analysis: these techniques separate cells for analysis or sorting.
Finding the right laboratory with the set of competencies required for cells, tissue, and animal imaging can be difficult.
Labtoo can look for any type of imaging services, for the development of new drugs and the development of diagnostics assays.
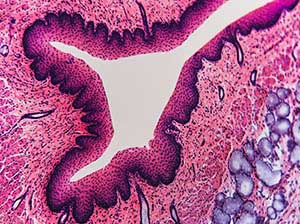
Select your Histology, Immuno-histochemistry and Anatomo-pathology Service
Histology refers to the microscopic study of tissues and their cellular composition. For this purpose, different labeling techniques are used, such as staining or the use of antibodies. Histology is a technique widely used in the diagnosis of abnormal cells, pathological anatomy or anapath, and the distribution of markers.
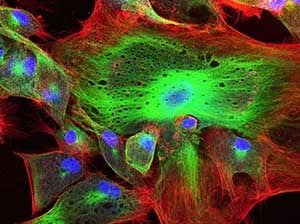
Select your Confocal and electron microscopy Service
Microscopy is a set of imaging techniques at the microscopic scale. Different techniques can be used, such as confocal microscopy (fluorescence microscopy) or transmission and scanning electron (electron beam) microscopy.
Select your Flow cytometry and Cell sorting Service
Cytometry is a technique for studying cells suspended in a sample. It is used in immunology because it allows the labeling of numerous biomarkers simultaneously, up to several dozen with mass cytometry. Cell sorting, adapted from flow cytometry, allows cells to be recovered according to predefined criteria.
Our team will handle your R&D service management from the beginning to the end
Perform a feasibility study by looking for existing expertise available within the network of partners
Set up a study protocol, financial quotation and preparing contracts with lab partners
Implement the study plan into a schedule, collect all needed materials and reagents and execute the service
Do you need more information about in vitro imaging services and technologies?
The different types of microscopes
There are multiple types of microscopes, here are the different types used specifically in biology.
Stereoscope
The stereoscope, also known as a dissecting microscope and stereomicroscope, is a light-illuminated microscope that provides a three-dimensional view of a specimen. It uses two eyepieces at different angles.
Stereoscopes have lower power than compound microscopes. Images are magnified only up to about 100 times, and their application is limited to growth monitoring in cell culture, or basic imaging.
Compound
A compound microscope is an instrument for viewing enlarged images of small objects on a glass slide. It can achieve higher magnification levels than stereo microscopes (up to 2000x) or other low-power microscopes and reduce chromatic aberration. Applications remain relatively limited: direct observation without a coupled photographic system.
Confocal
Confocal microscopes are also microscopes using light. Confocal microscopes allow to magnify specimens strongly with three-dimensional images. They also have higher resolutions, capable of differentiating details up to 120 nanometres from each other. The most common type of confocal microscope is the fluorescent microscope. This microscope uses intense light to excite the molecules in a specimen. These molecules emit light, or fluorescence, which is observed, allowing for higher magnification and resolution.
Transmission electron microscope
The first electron microscope was a transmission electron microscope (TEM) invented in Germany in 1931 by Max Knoll and Ernst Ruska. If light microscopes can magnify up to 1000x or 2000x at best, then the electron microscope magnifies objects up to 10,000x. A TEM works by focusing a single energy electron beam strong enough to pass through a very thin sample. The resulting images are then viewed by electron diffraction or direct electron imaging.
Scanning electron microscope
The scanning electron microscope (SEM) was invented in the early 1930s, and the development of the first commercial system was in 1965 by the Cambridge Instrument Company. The SEM works by scanning the surface of a sample with an electron beam. This beam creates different signals, secondary electrons, X-rays, photons, and others, all of which help to characterize the sample. The signals are displayed on a screen that maps the material properties of the sample.
Anatomopathology and Histology
Pathological anatomy, colloquially called "anapath", and officially called pathological anatomy and cytology (PAC) or Pathology, is based on the diagnosis of lesions based on their morphological appearance.
In concrete terms, this means making a diagnosis based on a sample of cells or tissues. For this, the specialist doctor will use histological or cytological methods, followed by microscopic observation.
Depending on the markers of interest to be studied, histology is based on different techniques: specific staining of cellular components, immunology to detect certain proteins, in situ hybridization to detect nucleic acids.
Commonly used in anatomopathology, particularly in oncology, histology is at the crossroads of many research projects: in research and "discovery" during the analysis of biomarkers on tissues, during preclinical studies on small animals, and throughout clinical studies.
Types of providers
Academic structures
Cytometry and microscopy require access to state-of-the-art instruments, and university facilities are able to provide such equipment and services.
Service companies
Some specialized structures may offer microscopy studies, especially for high-content screening (HCS) or other projects.
Freelance firms and experts
Small consulting firms offer histology services, and freelancers can carry out anapath studies.
Mass cytometry: what is it?
Unlike conventional or spectral flow cytometry, which is based on fluorescence, mass cytometry (MCM, CyToF), which emerged in the 1990s, is based on an analysis of the atomic mass of molecules.
Fluorophores that used to be coupled to fluorochromes are now coupled to non-radioactive metal isotopes.
Thus, 135 detection channels ranging from 75 to 209 daltons are available. Each channel can be assigned to an isotope, which will be coupled to an antibody or molecule targeting the molecules of interest.
The advantage of mass cytometry is therefore the wide availability of elements that can be used to mark cells: whereas classical cytometry is limited to a few markers used at the same time (largely due to the wavelength overlap of fluorophores and the background noise of the samples), MCM is free from these constraints. This also makes it possible to study rare events and to standardize analyses from quantifiable standards.
Finally, the MCM has a major advantage: sample preparation and study planning are done in a similar way (or sometimes even simplified!) compared to conventional cytometry. This facilitates method transfer and avoids having to start from a scratch.

